Aminoguanidine Hydrochloride
Product name:carbazamidine hydrochloride; (diaminomethylidene)hydrazinium chloride
Molecular Formula:CH6N4HCL
CAS:1937-19-5
Molecular Weight:110.55
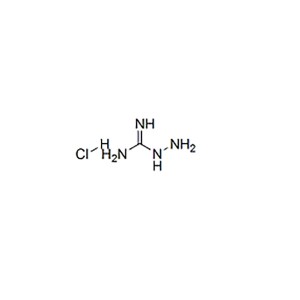
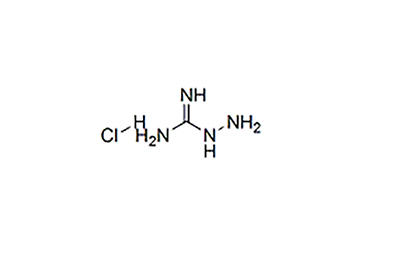
Structural Formula:
Use:Pharmaceutical, veterinary drugs
|
Index name |
Index Value |
|
|
Appearance |
White like crystalline powder |
|
|
Content |
≥98% |
≥99% |
|
Insoluble Substances |
≤0.2% |
≤0.1% |
|
Loss on Drying |
≤1.5% |
≤1% |
|
Ignition Residue |
≤0.2% |
≤0.1% |
|
Iron Content(Fe) |
10 ppm |
6 ppm |
|
Free Acid |
≤0.8% |
≤0.5% |
preparation
Preparation of aminoguanidine hydrochloride: 9 g of aminoguanidine carbonate was put into a 250ml three port flask, 20ml of absolute ethanol was added, the solid was insoluble in anhydrous ethanol to form a suspension. Under stirring at room temperature, the mixture of 6ml 30% concentrated hydrochloric acid and 10ml of absolute ethanol was dropwise added until there was no bubble, and then stirring reaction was continued at room temperature for 1 hour. The obtained suspension was heated to dissolve the solid completely, and then it was naturally lowered to room temperature. After being moved to the refrigerator and placed overnight, the white rod-shaped crystal with melting point of 166-167 ℃ was obtained.
Application
Daidzein has a variety of pharmacological effects, prevention and treatment of a variety of diseases. Cn200910144204.1 reported that aminoguanidine hydrochloride can be used to prepare a chemically modified daidzein, namely, daidzein 7,4 ‘- oxy aminoguanidine acetate. The soybean aglycone 7,4 ‘- oxy aminoguanidine acetate is a prodrug compound, which releases the parent drug – daidzein under physiological conditions, and improves its water solubility by covalent binding.
The preparation of Daidzein 7,4 ′ - o-aminoguanidine acetate: 0.5g aminoguanidine hydrochloride was dissolved in 100ml acetone, 0.02g phase transfer catalyst, 0.5g anhydrous potassium carbonate, 0.5g 7,4 ′ - chloroacetyl daidzein and 0.02g I2 were added into the solution. The reaction was carried out at room temperature for 24 hours. Acetone was evaporated from the filtrate under reduced pressure, and then separated by silica gel column. The eluent was ethyl acetate ∶ petroleum ether When the ratio is 1 ∶ 2, the white powder solid is obtained.